Product Name: Vanadium Nitride (VN)
Specification: 0.8-10um (D50)
Appearance: Irregular
Color: Black
Features: high hardness, high melting point, high conductivity, wear resistance, low coefficient of thermal expansion, high thermal conductivity, good thermal and chemical stability
Usage: In the fields of batteries, catalysts, photocatalysis, optoelectronics, advanced materials, etc

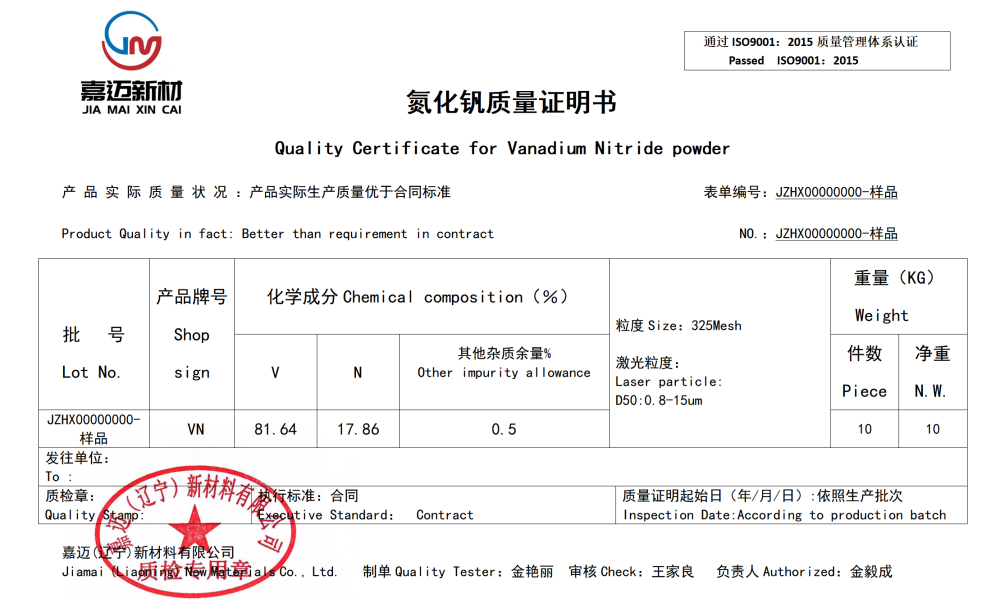

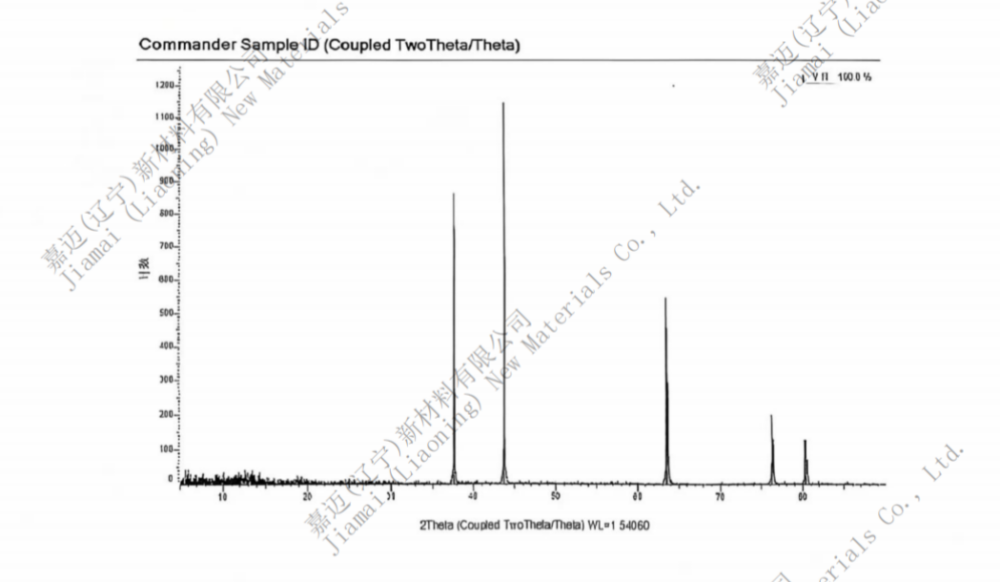

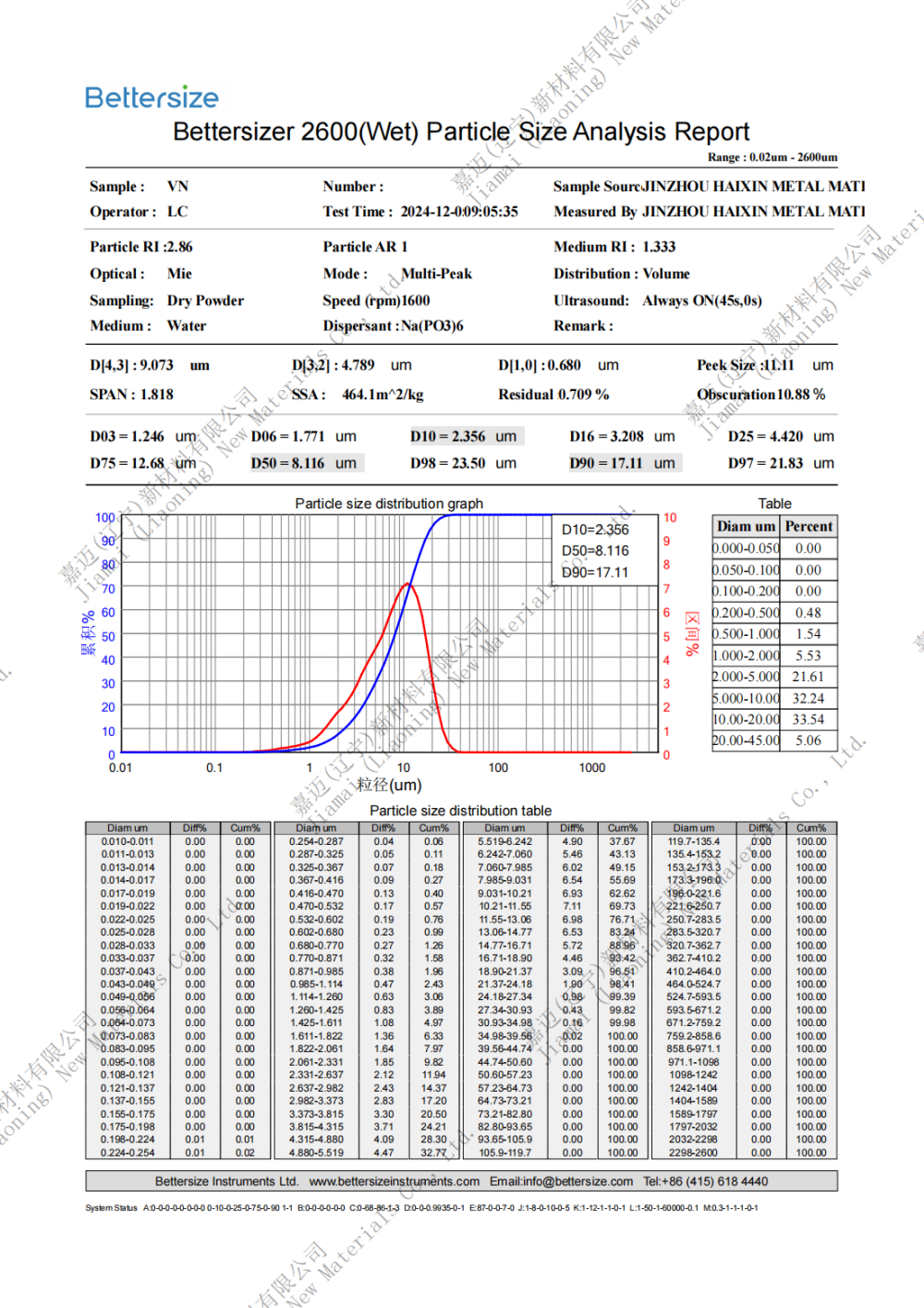

VANADIUM NITRIDE
Appearance: Black cubic crystalline powder
Density (g/mL, 25/4 ℃): 6.13
Melting point (º C): 2360
Electrical resistivity (μ Ω• cm): 85
Thermal conductivity (W/(m • K)): 11.3
Coefficient of body expansion (K-1): 8.1 × 10-6
Microhardness (kg/mm2): 1310
Synthesis method:
1. Direct synthesis method: Vanadium powder is reacted with nitrogen at high temperature to generate vanadium nitride.
2. Gas deposition method: By transferring a mixture of vanadium and hydrogen gas to the deposition substrate at high temperature, a vanadium nitride thin film is formed on the substrate.
3. Sol gel method: mix vanadium salt with the solution of corresponding nitriding agent to form gel, and then obtain vanadium nitride through drying, roasting and other steps.
Purpose:
1. Steel industry: efficient microalloying additives
Vanadium nitride (VN), as a new type of alloy additive, can replace traditional vanadium iron and significantly improve the performance of steel
Strengthening the performance of steel: Adding vanadium nitride can promote the precipitation of vanadium, refine grain size, improve the strength, toughness, ductility, and thermal fatigue resistance of steel, while improving weldability and formability.
Typical applications: oil/gas pipelines, bridges, railway tracks, automotive structural components, and other scenarios that require high strength and durability.
2. Wear resistant and protective coatings
The high hardness (microhardness of about 1310-1520HV) and corrosion resistance of vanadium nitride make it an ideal surface coating material.
3. Welding
The nitrogen component in vanadium nitride can promote the precipitation of vanadium after hot processing, resulting in finer precipitate particles and better improvement of the weldability and formability of steel. It can be used to produce high-strength welded steel bars, non quenched and tempered steel, high-speed tool steel, high-strength pipeline steel and other high-strength low-alloy steel products.
4. Hard alloy
Used as a raw material for hard alloys, it is a new and efficient additive for vanadium alloys.
Storage method: This product should be sealed and stored in a dry and cool environment. It should not be exposed to air for a long time to prevent moisture from causing aggregation, affecting dispersion performance and usage effect. In addition, it should be avoided from heavy pressure and not in contact with oxidants. It should be transported as ordinary goods.