Chinese name: Molybdenum carbide
Chinese name: Molybdenum carbide
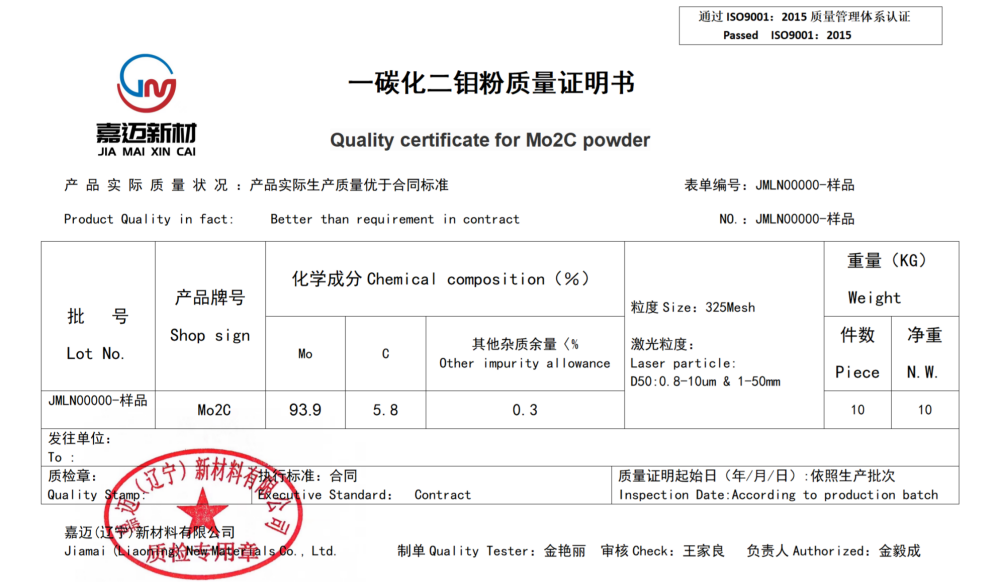
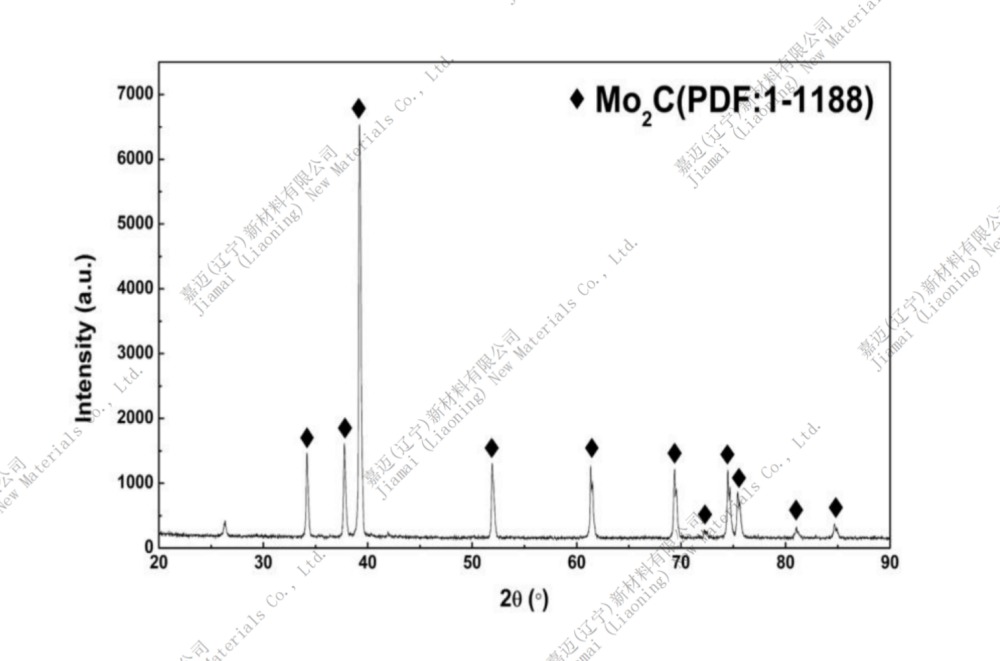
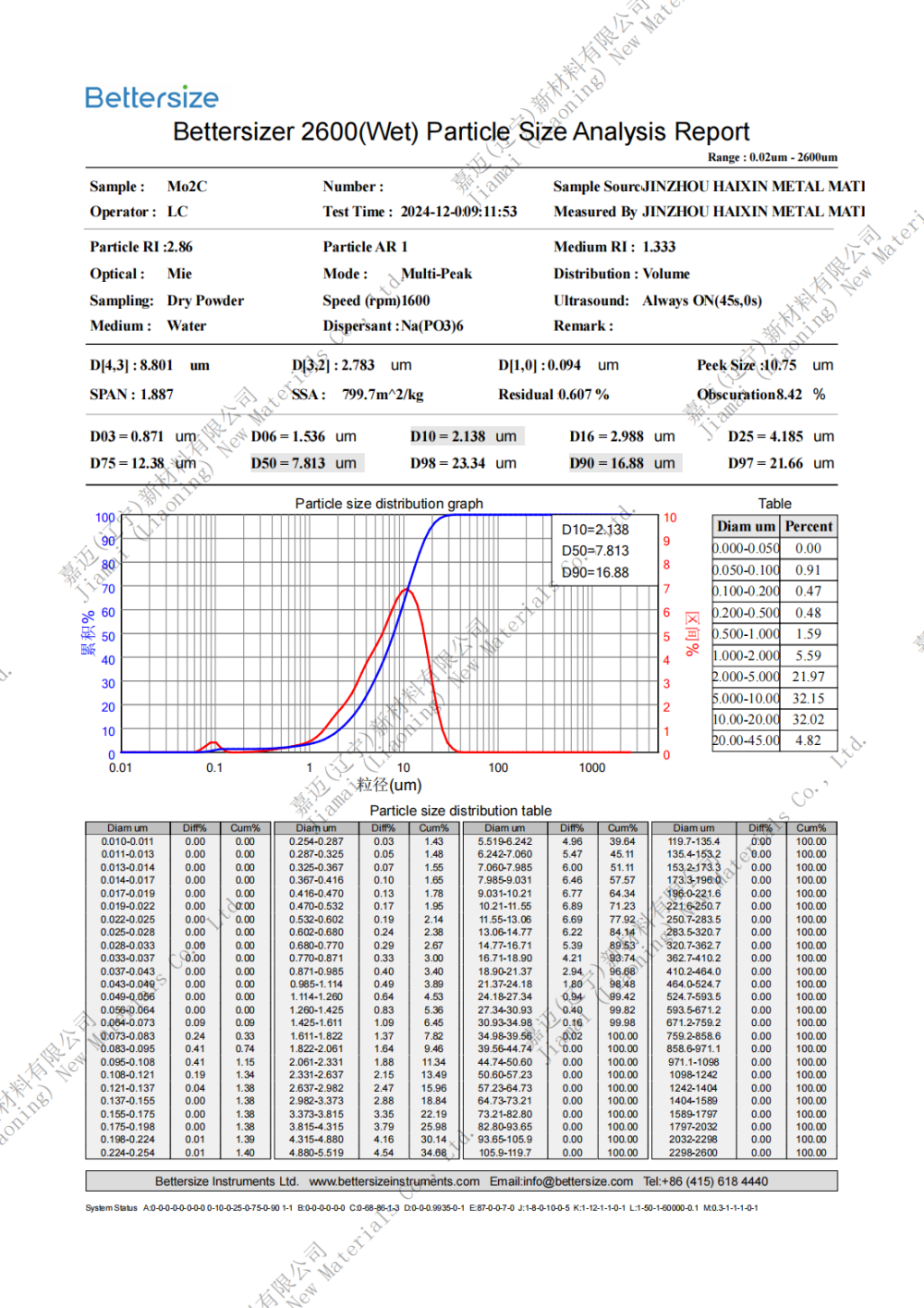
Chemical formula: Mo2C
English: Molybdenum Carbide
CAS: 12011-97-1
Melting point: 2615
Appearance: grey and black
Molecular weight: 204
EINECS: 234-569-3
Density: 9.18g/cm3
Boiling point: 2690
Water-soluble: insoluble




At present, the chemical methods for preparing molybdenum carbide mainly include compound pyrolysis method, programmed temperature reaction method, high temperature carbon reduction method of molybdenum oxide and gas phase reaction of metal molybdenum compounds with certain volatility. Currently widely used is the temperature programmed reaction method (TPRe), the transition metal oxide and carbon source and hydrogen mixture as raw materials, through a process similar to the temperature programmed reduction process, the transition metal oxide through the reduction and carbonization (carburizing) reaction process at a set temperature. Commonly used carbon source substances are methane, ethane, ethylene or carbon oxides (CO, CO2), etc., and the reaction temperature is generally controlled at 400 ~ 1000℃. Liang et al. synthesized nanometer molybdenum carbide by temperature-programmed reduction method using carbon material with high surface area as carbon source. 1. MoO3 was prepared by the process of heating and decomposing ammonium molybdate at 500℃. Supported molybdenum trioxide precursor can be prepared by impregnating alumina carrier in ammonium molybdate and ammonia solution, stirring for 0.5h, leaving at room temperature for 24h, evaporating in water bath at 80 ~ 90℃, drying at 115℃ for 12h, roasting at 500℃ for 4h. Molybdenum carbide was prepared with hexane as carbonizing material by temperature programmed method. The programmed heating process is as follows: from room temperature to 300℃, the heating rate is 10℃/min; From 300℃ to 600℃, the heating rate is 1℃/min; Constant temperature at 600℃ for 2h. 2. Gas phase method Gas phase method generally uses high specific surface activated carbon (specific surface area greater than 200m2/g) and transition metal volatile compounds, stoichiometric ratio into the flow of the reactor, reaction at 900 ~ 1400℃ for a certain time, in the inert gas cooling and recovery of carbide process. The mixture of high specific surface carbon and MoO3 was heated to 800℃ in argon, so that MoO3 sublimed and adsorbed on carbon, and then heated to 1300℃ for a certain time to prepare Mo2C. It has been reported that Mo2C of the six-crystal system can be obtained by the reaction of MoO3 and 1150m2/g carbon with a C/Mo molar ratio of 6:1, and its specific surface can reach 213m2/g. Leclercq et al. vaporized volatile metal compounds into containers containing low-pressure hydrocarbons, and directly carbonized them at high temperatures to produce carbides. Different carbides and carbon oxides are prepared by changing the gas composition in the reactor and the types of metal compound precursors. Nagai et al. used this method to prepare Mo2C/Al2O3 catalyst with 3 times higher activity than the impregnation method under 973K vacuum. However, the disadvantage of this method is that the conditions are not easy to control and the synthesis amount is small. 3. Thermal decomposition method Thermal decomposition method refers to the process of using transition metal oxides or halides to react with the specified organic matter to form metallic organic matter, and then the organic matter is thermal decomposition reaction under an inert atmosphere. Bayer uses it to react with organic compounds containing two hydroxyl groups, remove excess organic compounds, and decompose under vacuum or inert gas to form metal carbides. Sehwartzkopf et al. carbonized metal oxide powders directly at high temperatures, but the resulting sample had a low specific surface area. At present, the preparation of molybdenum carbide is developing towards the practical type with high specific surface and high dispersion. 4. Liquid phase reaction method Low temperature liquid phase reaction method refers to a series of substances dissolved in a suitable solvent, so that it can undergo a chemical reaction under relatively mild conditions.
2. Application field:
1, cutting tools: molybdenum carbide has extremely high hardness and wear resistance, so it is widely used in the manufacture of high hardness cutting tools, such as drills, milling cutters, turning tools, etc.
2, electronic materials: molybdenum carbide has low resistivity, good thermal stability, and small thermal expansion coefficient, so it can be used as semiconductor materials and energy storage materials. Molybdenum carbide has good electrical conductivity and high temperature stability, so it is widely used in the manufacture of electronic materials, such as electrodes, capacitors, etc.
3, aerospace: molybdenum carbide has good corrosion resistance and high temperature stability, so it is widely used in the aerospace field, such as manufacturing engine components, combustion chambers, etc.
4, molybdenum carbide can also be used as a coating material, can effectively improve the surface hardness and corrosion resistance, for the manufacture of acid and alkali resistance, corrosion resistance and other special coatings.
5, molybdenum carbide catalytic performance: It has been found that molybdenum carbide has a high initial catalytic activity in benzene hydrogenation reaction, although the catalyst is gradually deactivated with the reaction, but its steady-state conversion frequency (TOF) can still be compared with precious metals Pt or Ru. The catalytic activity of α-MoC-x for thiophene hydrodesulfurization at atmospheric pressure and 300℃ is similar to that of molybdenum sulfide, a common hydrodesulfurization catalyst. Molybdenum carbide catalyst main catalytic reaction types: (1) hydrogenolysis reaction; (2) Hydrodesulfurization HDS and hydrodenitrification HDN reaction; (3) isomerization reaction; (4) hydrocarbon conversion and synthesis reactions; (5) Application of ammonia synthesis.
Third, storage method:
This product should be sealed and stored in a dry, cool environment, should not be exposed to the air for a long time, prevent moisture agglomeration, affecting the dispersion performance and use effect, should also avoid heavy pressure, do not contact with oxidants, according to the general cargo transport.