
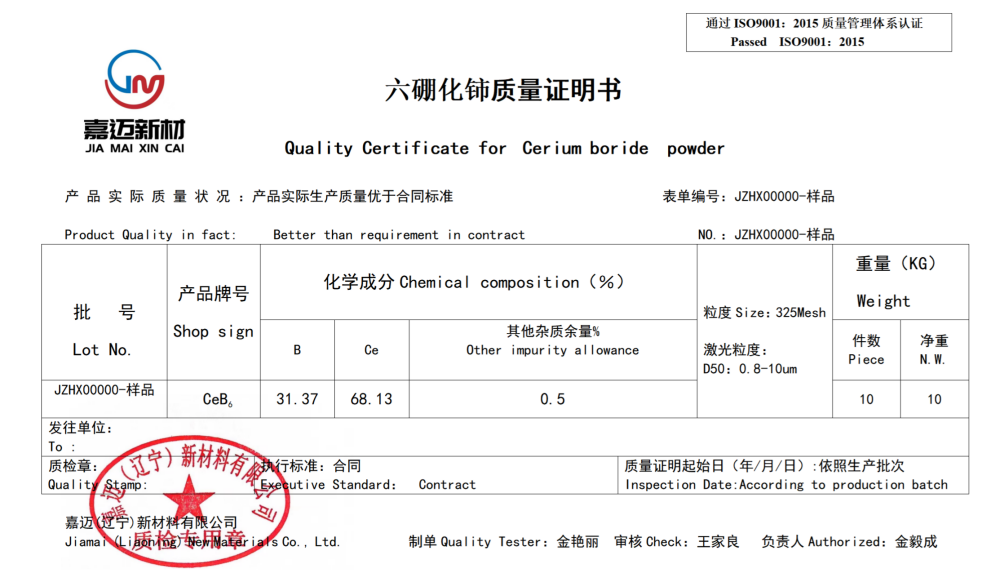
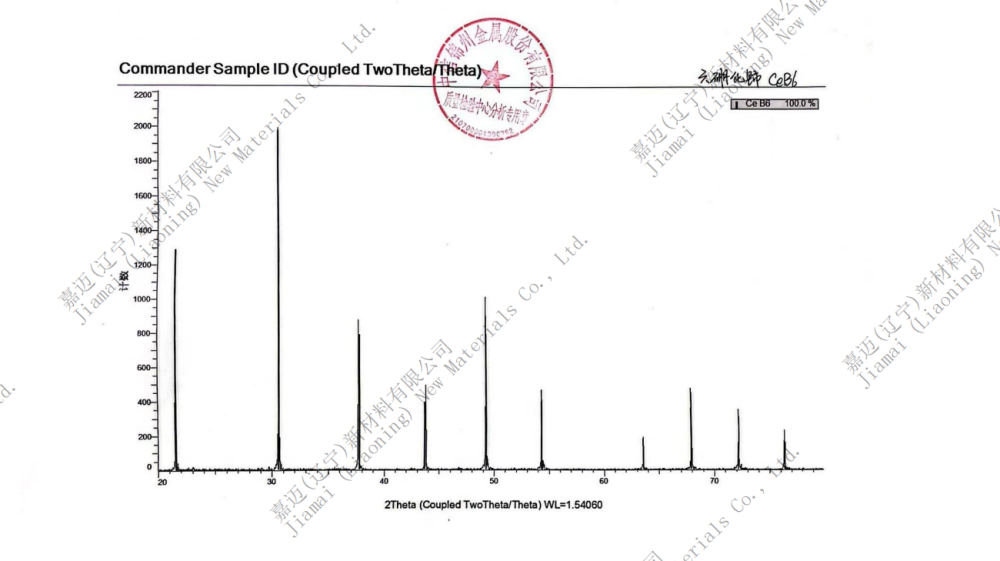
Product Name: Cerium Hexaboride (CeB6)
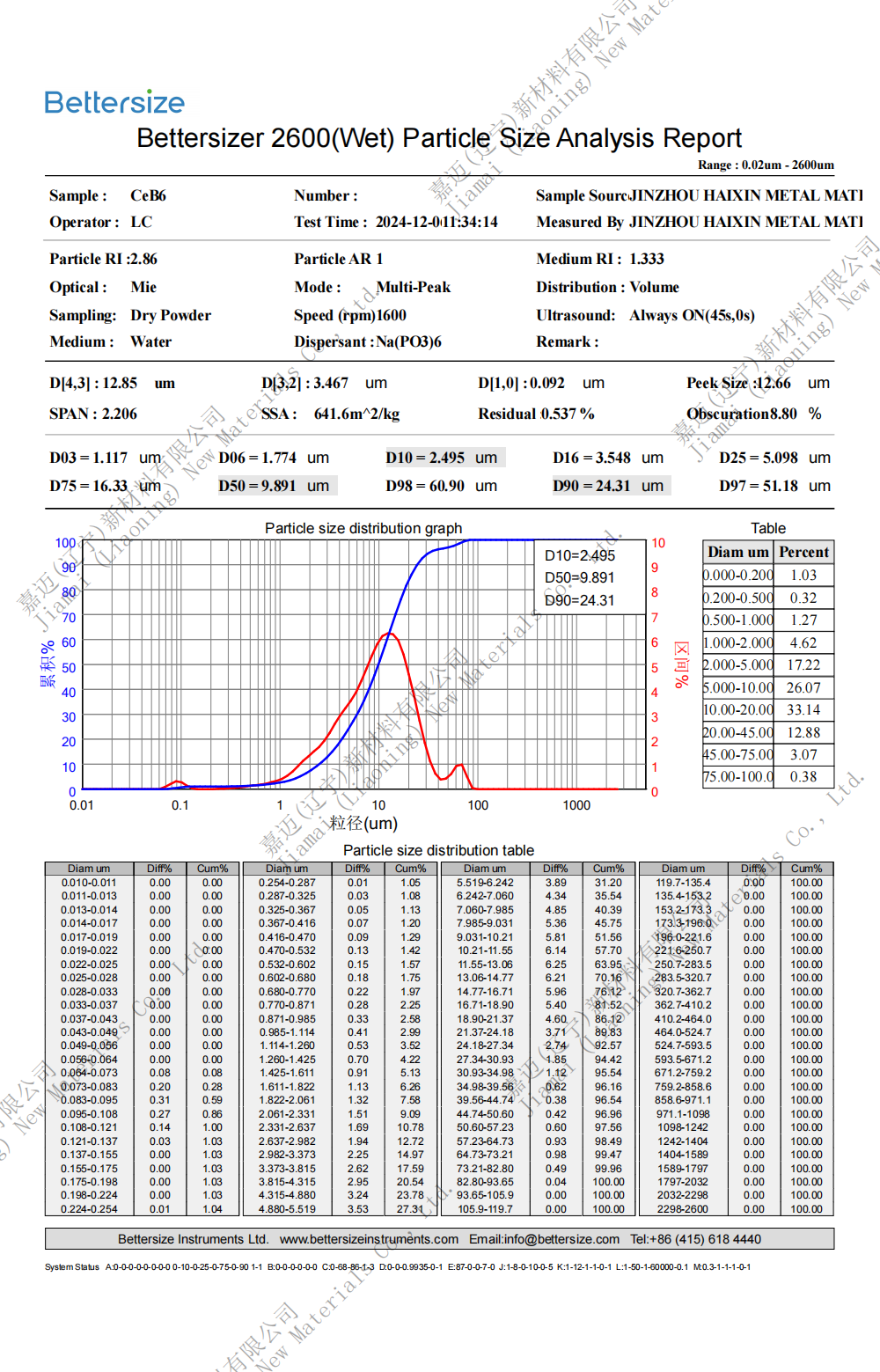
Specification: 0.8-10um (D50)
Appearance: Irregular
Color: Black Grey
Features: high melting point, high current emission density, uniform emission, strong anti poisoning ability
Application: In fields such as electron microscopy, electron beam etching, electron beam welding, X-ray tubes, and free electron lasers




Cerium hexaboride
Foreign name: CeB6
Density: 4.80 g/cm3
Melting point: 2552 ° C
Working temperature: 1450 ° C
definition:
Cerium boride is an inorganic compound with the chemical formula CeB6, composed of cerium and boron elements. Cerium hexaboride (CeB6) has a CsCl structure (belonging to a simple cubic crystal system, with large spheres representing Ce elements), but the difference is that B6 octahedral clusters occupy the Cl position and Ce occupies the Cs position. It is a refractory ceramic material with a low work function, making it one of the known cathode materials with the highest electron emissivity, and it is also very stable in vacuum.
Crystal structure:
Cerium boride has a special crystal structure and belongs to the cubic crystal system. Each cerium atom is surrounded by six boron atoms, forming an octahedral structure. The stable structure and unique electronic structure of cerium boride crystals provide a foundation for their application in materials science.
nature:
Cerium boride is a black crystal with a metallic luster.
2. It has good conductivity and is a high-temperature superconducting material.
3. Cerium boride has a high melting point of approximately 2300 degrees Celsius.
4. It has high hardness and wear resistance.
5. Cerium hexaboride typically operates at a temperature of 1450 ° C. Cerium hexaboride, like lanthanum hexaboride, slowly evaporates during cathode operation. The CeB6 cathode can maintain its optimal shape for a longer period of time at an operating temperature of 1850 K, resulting in a longer lifespan. At the same time, due to its evaporation rate being about 30% slower than lanthanum boride, the hot cathode coating of cerium boride is also more difficult to remove than lanthanum boride.
Purpose:
Cerium boride is commonly used in electronic devices, electronic vacuum devices, and materials for electrical shock activity in high-temperature environments.
2. It can also be used as an additive for vehicle emission control catalysts to enhance their activity and stability.
Cerium boride also has applications in some optical fields, such as optical components for high-intensity lasers.
4. Cerium hexaboride is mainly used as a coating for hot cathodes, or the hot cathode is directly composed of cerium hexaboride crystals. Cerium hexaboride (CeB6) and lanthanum hexaboride (LaB6) are often used as high current hot cathode coatings. Hexaborides have a low work function, around 2.5 eV, and also have a certain tolerance to cathode contamination. Cerium boride cathodes have a lower evaporation rate than lanthanum boride at 1700 K, but they become consistent at 1850 K and even have higher evaporation rates above that temperature. Cerium boride cathode has a lifespan 50% longer than lanthanum boride due to its higher resistance to carbon pollution. The brightness of boride cathodes is ten times that of tungsten cathodes, and their lifespan is 10-15 times that of tungsten cathodes. Some laboratory experiments have shown that CeB6 is more tolerant to the negative effects of carbon pollution than LaB6. They are commonly used in applications such as electron microscopes, microwave tubes, electron beam etching, electron beam welding, X-ray tubes, and free electron lasers.
CeB6 is also a very stable material, resistant to pollution and degradation, which ensures its performance remains consistent over time. In addition to its performance advantages, CeB6 is also a cost-effective electron source, making it a viable option for a wide range of SEM applications. These applications include imaging, material analysis, and spectroscopy.
One of the main benefits of using CeB6 as an electron source is its high brightness. Brightness is an indicator of the number of electrons emitted per unit area per unit time, and high brightness is crucial for obtaining high-quality SEM images. The excellent brightness of CeB6 provides a higher signal-to-noise ratio, offering better image resolution and quality compared to other electronic sources such as tungsten wires. By using CeB6, more current and smaller spot size can be utilized, resulting in higher resolution than tungsten. In addition, compared to other electron sources, CeB6 has a longer lifespan, which reduces maintenance and downtime.
Manufacturing method:
Cerium boride can be prepared by the following methods:
1. Direct reduction method: Reacting cerium and boron at high temperature to produce cerium boride.
2. Thermal reduction method in solution: reacting cerium salt with boron in solution to produce cerium boride.






