Product Name: Lanthanum Hexaboride (LaB6)
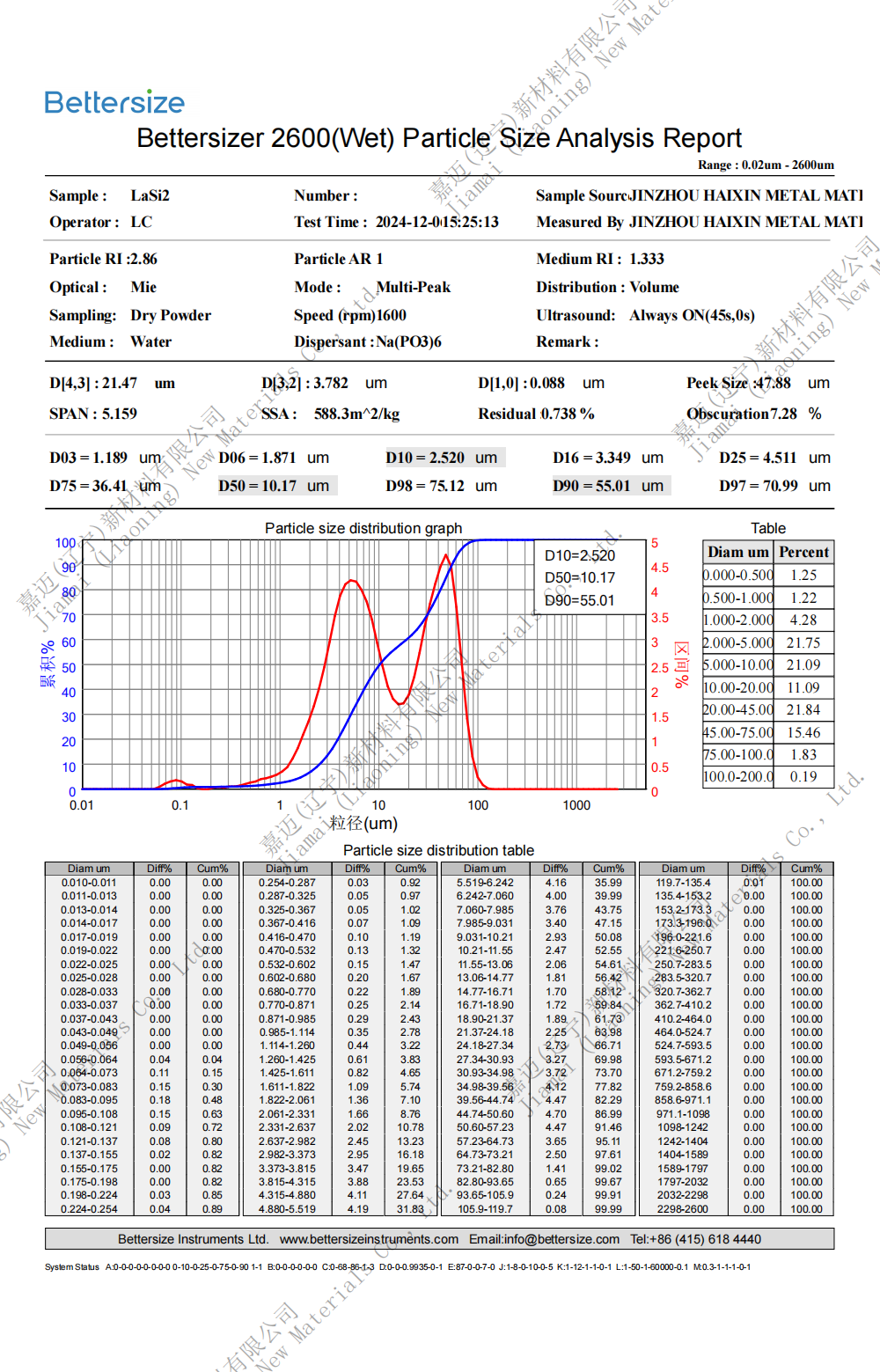
Specification: 0.8-10um (D50)
Appearance: Irregular
Color: Purple
Features: high melting point, excellent thermal radiation and microhardness, high electron emissivity
Application: Aerospace, nuclear fusion reactors, high-temperature coating materials, instruments and equipment, medical devices, metallurgy and other fields



English name: Lanthanum hexaboride
CAS number: 12008-21-8
Molecular formula: B6La
Molecular weight: 203.7715
Density (g/mL, 20 ℃): 4.76
Mohs hardness: 9.5
Room temperature resistance: 15-27 μ Ω
Vickers hardness: 27.7GPa
Work function: 2.66eV
Emission constant: 29A/cm2 · K2
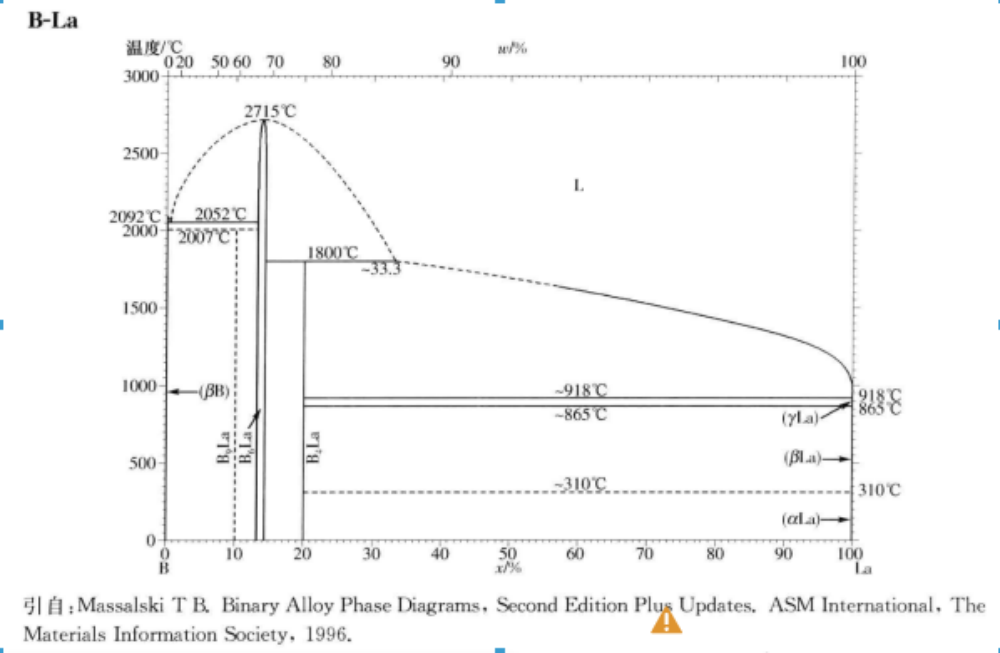
Melting point (º C): 2715
CAS number: 12008-21-8
MDL Number: MFCD00151350
EINECS number: 234-531-6
PubChem Number: 24854496
Solubility: Difficult to dissolve in water or hydrochloric acid. Chemical properties: Very stable and does not react with water, oxygen, or even hydrochloric acid; At room temperature, it only reacts with nitric acid and aqua regia; Oxidation only occurs at 600-700 ℃ in an aerobic atmosphere. In a vacuum atmosphere, LaB6 material is prone to react with other substances or gases, forming low melting point substances; At high temperatures, the formed substances will continuously evaporate, exposing the low work function surface of lanthanum hexaboride crystals on the emission surface, thereby endowing lanthanum hexaboride with excellent anti poisoning ability.
The crystal structure of borides determines a series of unique properties:
1) Due to the strong bonding force between boron atoms (lattice constant 4.145 Å), it is a refractory compound with a melting point of 2210 ℃.
2) The chemical properties are very stable and do not react with water, oxygen, or even hydrochloric acid; At room temperature, it only reacts with nitric acid and aqua regia; Oxygen will only undergo oxidation at 600-700 ℃.
3) Within a certain temperature range, the coefficient of expansion approaches zero.
4) Good stability in air, surface contamination during use can be restored by vacuum heat treatment.
5) Good resistance to ion bombardment and able to withstand high field strength.
6) Due to the absence of valence bonds between metal atoms and boron atoms, the valence electrons of metal atoms are free. So borides have high conductivity, and the resistance of lanthanum hexaboride is roughly the same as that of lead metal. The temperature coefficient of its resistivity is positive.
7) If hexaborides are brought into contact with refractory metals at high temperatures, boron will diffuse into the metal lattice and form interstitial boron alloys with the metal. At the same time, the boron framework will disintegrate, allowing the metal atoms to evaporate.
8) When borides are heated to a considerable temperature, the metal atoms on the crystal surface evaporate, but are immediately replenished by metal atoms diffusing from within the lattice, while the boron framework remains unchanged, minimizing the loss of surface active substances.
Synthesis method:
Mix and stir a small amount of lanthanum oxide, boron, and carbon black powder (about 1g in total) according to stoichiometry until uniform, and press the reactants into thin sheets [1in (0.0254m) × 1/2in × 1/16in]. The thin sheet is loaded into a graphite crucible with a height of 1.5 inches and a diameter of 1.7 inches. The latter is then placed in a larger graphite crucible (4 inches high and diameter), filled with hydrogen gas, and heated at 1500 ℃ for 2 hours or 1800 ℃ for 1 hour.
Structural properties:
Due to the need for two electrons to stabilize the structure of the B6 octahedral network, one of La's three valence electrons will be abundant, and LaB6 behaves as a metallic conductor. The surface plasmon resonance absorption of metal conductor LaB6 nanomaterials is around 1000nm, which can compensate for the poor blocking effect of infrared barrier materials such as ITO (indium tin oxide) in the near-infrared region, making LaB6 an important infrared barrier material. Due to the strong covalent bonds between B atoms in the LaB6 structure, a tight spatial network is formed, which gives it low volatility, low work function, high melting point, high strength, and high stability.
Usage: Widely used, it has been successfully applied in more than 20 military and high-tech fields such as radar aerospace, electronics industry, instrumentation, medical equipment, home appliance metallurgy, environmental protection, etc. 1) Electron emission cathode. Due to the low work function of electrons, cathode materials with the highest emission current at medium temperatures can be obtained, especially high-quality single crystals, which are ideal materials for high-power electron emission cathodes. 2) High brightness point light source. Core components used for preparing electron microscopes, such as optical filters, soft X-ray diffraction monochromators, and other electron beam light sources. 3) High stability and long lifespan system components. The excellent comprehensive performance enables it to be applied in various electron beam systems, such as electron beam engraving, electron beam heat sources, electron beam welding guns, accelerators, and other engineering fields for producing high-performance components.
Packaging and storage: Sealed and stored in a dry and cool environment, not exposed to air, to prevent moisture from causing oxidation and aggregation, affecting dispersion performance and usage effectiveness