With the gradual depletion of traditional fossil fuels such as oil and coal and the worsening of environmental problems, the traditional energy structure system based on fossil fuels is facing unprecedented crises and challenges. Hydrogen has the advantages of high energy density, excellent combustion performance, and cleanliness without pollution, making it the best alternative green energy to traditional fossil fuels.
However, the utilization of hydrogen energy largely depends on the development of hydrogen production technology. Currently, industrial hydrogen production processes mainly include petrochemical catalytic cracking and natural gas steam reforming for hydrogen production. This process does not meet the development needs of "green and sustainable development" from the perspectives of environment and comprehensive energy utilization. In recent years, with the continuous development of new power generation technologies (such as solar power, wind power, nuclear power, hydropower, geothermal power, etc.) and the continuous optimization and upgrading of power grid systems, the advantages of electrolytic water hydrogen production technology have been continuously amplified, and even praised by many scientists and entrepreneurs as the "most ideal industrial hydrogen production method". The core issue of this technology is the development of efficient, stable, and clean hydrogen production (including oxygen evolution) electrocatalysts. At present, the most effective electrocatalyst for electrocatalytic hydrogen production process is platinum based catalyst, because this type of catalyst has the lowest overpotential and high stability in the electrolysis of water for hydrogen production. However, the high price and low storage capacity of platinum severely restrict the widespread application of this type of catalyst in hydrogen production through electrolysis of water and the significant development of this hydrogen production process. Therefore, finding inexpensive and replaceable high activity electrocatalytic hydrogen production catalysts is the core issue in developing hydrogen production processes. The latest research shows that pre transition metal carbides exhibit high catalytic activity and stability in electrocatalytic hydrogen production reactions. Among them, molybdenum carbide (MoxC) is one of the best alternative catalysts widely studied in recent years due to its high stability, simple synthesis method, and wide pH range,
Development Overview:
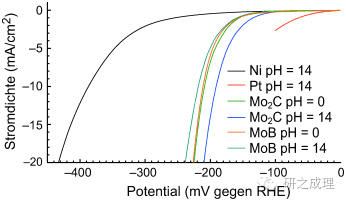
Since 2012, Professor Hu Xile's research group at the Swiss Federal Institute of Technology in Lausanne has used commercial B-MoxC for the first time in the field of electrocatalytic hydrogen evolution, marking the official beginning of the application of molybdenum carbide in electrocatalytic hydrogen production. Experiments have found that commercial β - Mo>C exhibits excellent precipitation activity under acidic (pH=0) and alkaline (pH=14) conditions, with a current density of 20mA/cm? At that time, the overpotentials were 240mV and 210mV, respectively.
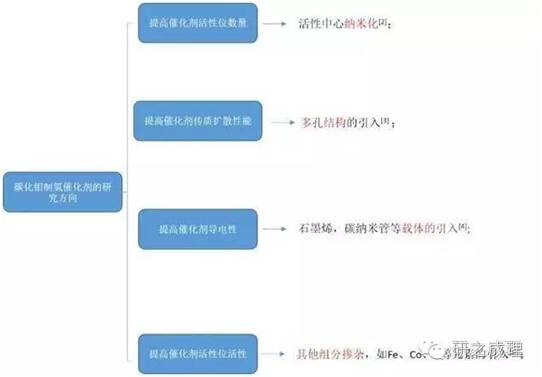
The application of molybdenum carbide materials in this field has achieved rapid development, and the main research can be divided into four directions: (1) improving the dispersibility of molybdenum carbide and using nanotechnology to expose as many active sites as possible in the electrocatalytic process; (2) Improve the porosity of catalyst materials to accelerate mass transfer and diffusion processes (electrolyte, hydrogen) during the catalytic process; (3) Due to the poor conductivity of molybdenum carbide, a significant amount of research has been conducted to improve the conductivity of the catalyst during electrocatalytic hydrogen evolution by introducing other conductive carriers, such as graphene, CNT, etc; (4) By doping, the electronic structure of molybdenum carbide can be altered to regulate its hydrogen evolution performance. Based on the rough classification of development directions and combined with the work done by the research group in this field, this article provides a brief review and evaluation of the synthesis of molybdenum carbide and its application in electrocatalytic hydrogen production.
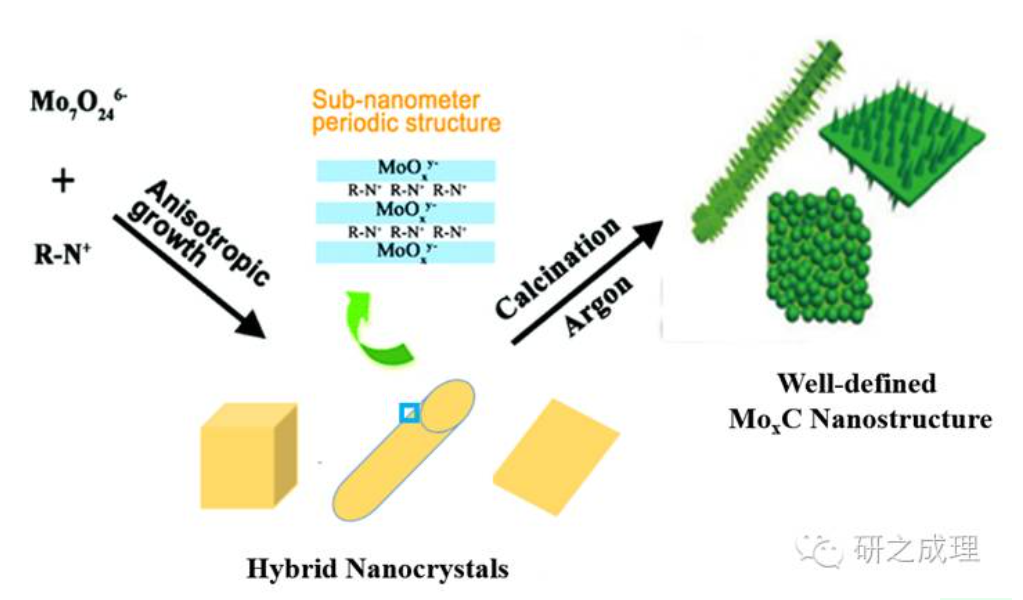
Platinum and other precious metals with similar d-electron structures are known as "precious metal catalysts" and can be used as hydrogenation/dehydrogenation catalysts. The traditional synthesis method of molybdenum carbide is the "gas-solid phase synthesis method", also known as MoO; Obtained through high-temperature carbonization in a CH4/H mixed gas environment. The controllability of this method is poor, and the synthesized molybdenum carbide particles have a large size (in the micrometer range). In addition, this method is highly risky and involves "gas-solid multiphase reactions", resulting in "longitudinal differences" in the synthesized catalyst. Our project team (led by Professor Tang Yi from Fudan University and Professor Gao Qingsheng from Jinan University) has developed a method for the secondary conversion of organic-inorganic hybrids, which can effectively control the synthesis of nanoscale molybdenum carbide catalysts. This method involves synthesizing organic-inorganic hybrid precursors containing Mo (nanocrystals with different morphologies, compositions, and structures), and then subjecting them to high-temperature carbonization to obtain highly dispersed molybdenum carbide catalysts. During this process, there is a "sub nano periodic structure" between the organic matter and the Mo containing component, and a quasi homogeneous carbonization reaction can occur throughout the entire material at high temperatures, overcoming the interface reaction drawbacks of gas-solid synthesis methods and achieving controllable synthesis of molybdenum carbide nanoparticles (~5nm). Among them, the nano molybdenum carbide nanowires synthesized by this method exhibit high activity in electrochemical precipitation, with a current density of 10mA/cm? At that time, the overpotential drop was only ca.130mv [2].
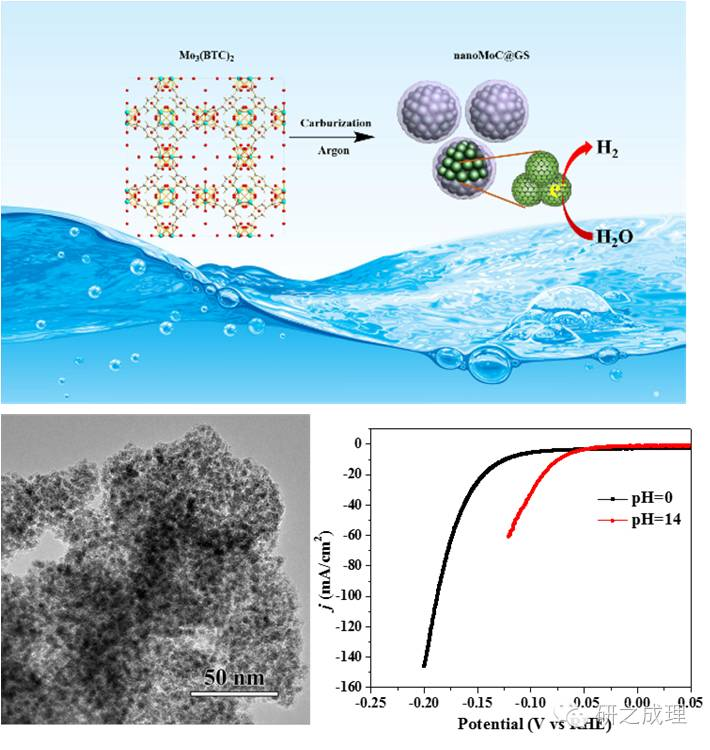
At present, the synthesis process of molybdenum carbide (MoxC) reported inevitably uses high temperature (~900 ℃), which inevitably causes sintering and agglomeration of MoxC particles. On the other hand, high synthesis temperatures can cause the collapse of the catalyst's pore structure, resulting in the catalyst generally having a smaller specific surface area (<50="">2/g). The above-mentioned technical bottlenecks severely restrict the exposure of active sites and the diffusion of reaction products and reactants in the electrocatalytic hydrogen production process of molybdenum carbide MoxC catalysts, greatly affecting the activity of this type of electrocatalyst. Our research group continues the method of "secondary transformation of organic-inorganic hybrids", using MOFs (MO; (BTC) 2) rich in organic matter as the hybrid precursor, which can be prepared by high-temperature transformation under Ar gas protection
Ultra dispersed nano MoC electrocatalyst (~3nm), with the active center MoC tightly embedded within the graphitized carbon layer. In addition, due to the introduction of a fluffy graphite elastic layer, the specific surface area of the electrocatalyst is as high as 187m/g. The catalyst exhibits extremely high catalytic activity under both acidic and alkaline conditions, with a current density of 10mA/cm? At that time, the overpotentials were 124mv and 77mV, respectively.
Due to the poor conductivity of molybdenum carbide itself, the electron transfer rate during the electrocatalytic process is also crucial for the reaction. So, our research group combined the method of "secondary conversion of organic-inorganic hybrids" with graphene with good conductivity to synthesize nano B-Mo and C electrocatalytic hydrogen evolution catalysts supported on reduced graphene. The electrochemical activity under acidic conditions is: current density of 10mA/cm? At that time, the overpotential drop was only ca.120mv [4].
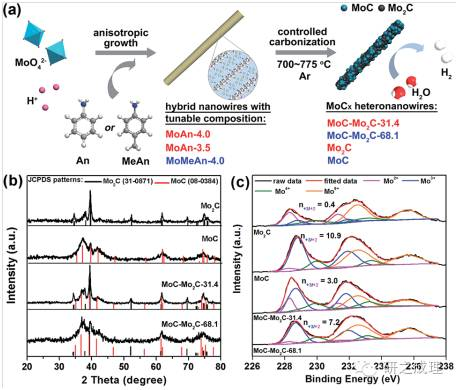
On the basis of discovering the high hydrogen evolution activity of nano molybdenum carbide [3,4], the project team further modified the composition of MoOx amine organic-inorganic hybrid nanowires and developed MoC Mo, C heterogeneous nanowires through a "controllable carbonization" strategy. Utilizing the synergistic effect on the MoC MozC nanointerface, while promoting proton reduction (Volmer step) and hydrogen adsorption desorption process (Heyrovsky/Tafel step), to enhance catalytic activity. In acidic and alkaline environments, the hydrogen evolution overpotentials (n10) of MoC Mo and C heterogeneous nanowires are 126mV, respectively, and the catalytic activity remains unchanged for more than 20 hours without significant degradation. This work provides a new method for modulating the electronic properties of molybdenum carbide active centers and optimizing hydrogen production kinetics, and provides a reference for further research on catalytic mechanisms [5].
Based on the control principle of the electronic structure of molybdenum carbide mentioned above, the project team continues to use Co, which has abundant d electrons, as a doping element to regulate the electronic structure of Mo2C. Co doped MoxC electrocatalysts can be obtained by introducing Co during the construction of MoOx amine organic-inorganic hybrid nanowire precursors, followed by high-temperature carbonization. Research has shown that the introduction of Co can increase the electron cloud density near the Fermi level of Mo and C, weaken Mo-H, and thus improve the hydrogen evolution performance of Mo and C. In acidic and alkaline environments, the hydrogen evolution overpotentials (n10) are 140mV and 118mV, respectively, and the catalytic activity remains stable for more than 20 hours without significant decay.





