Product Name: Iron Monoboride (FeB)
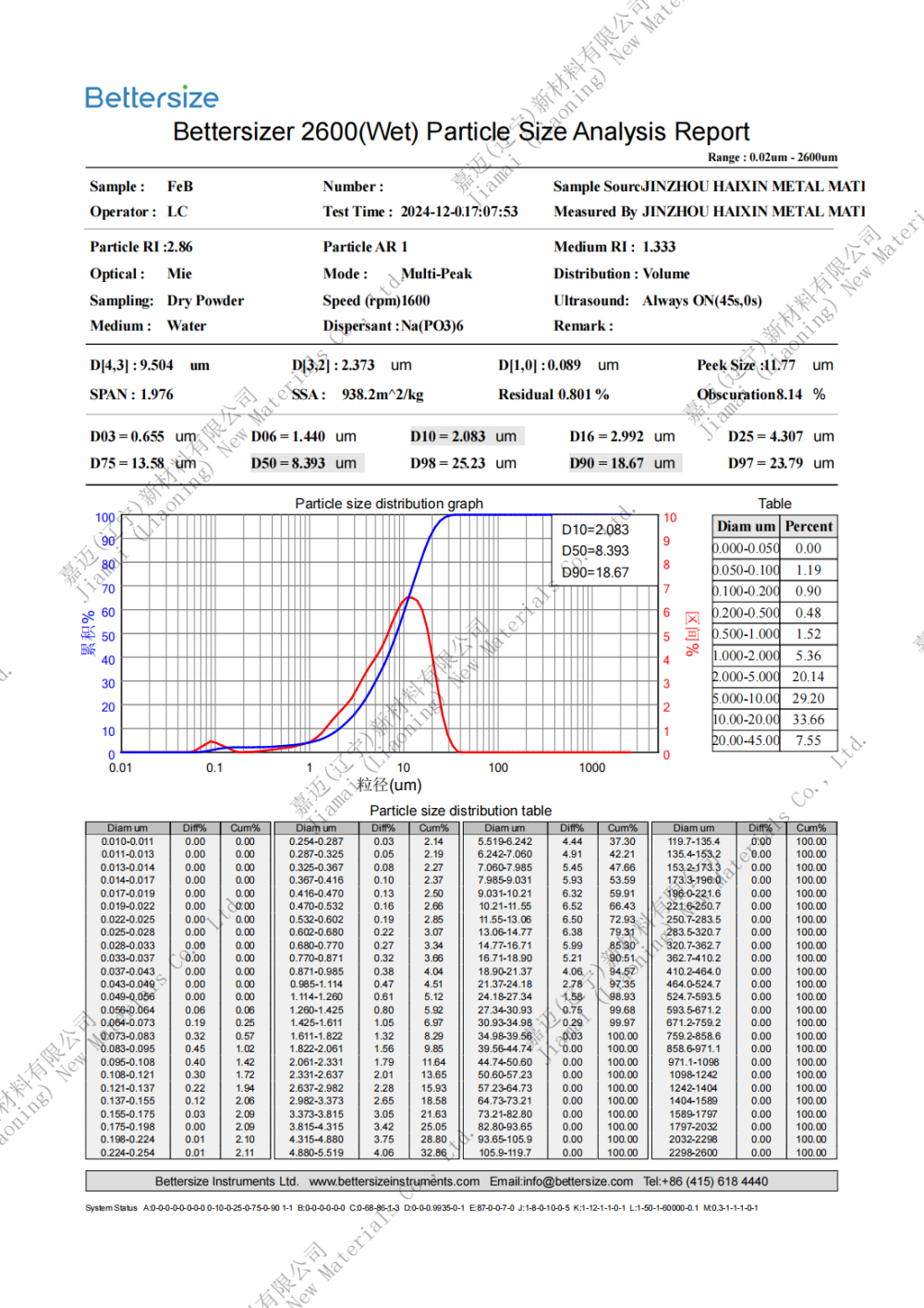
Specification: 0.8-10um (D50)
Appearance: Irregular
Color: Black Grey
Features: high melting point, high hardness, good wear resistance, high temperature resistance, conductivity, and magnetism
Application: Aerospace, industrial, magnetic materials, electrochemistry, materials science and other fields

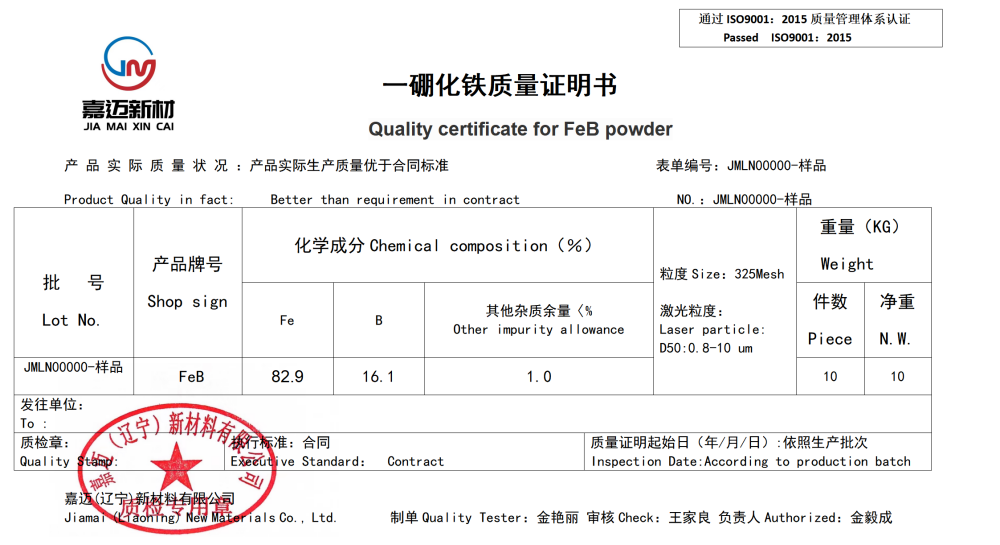

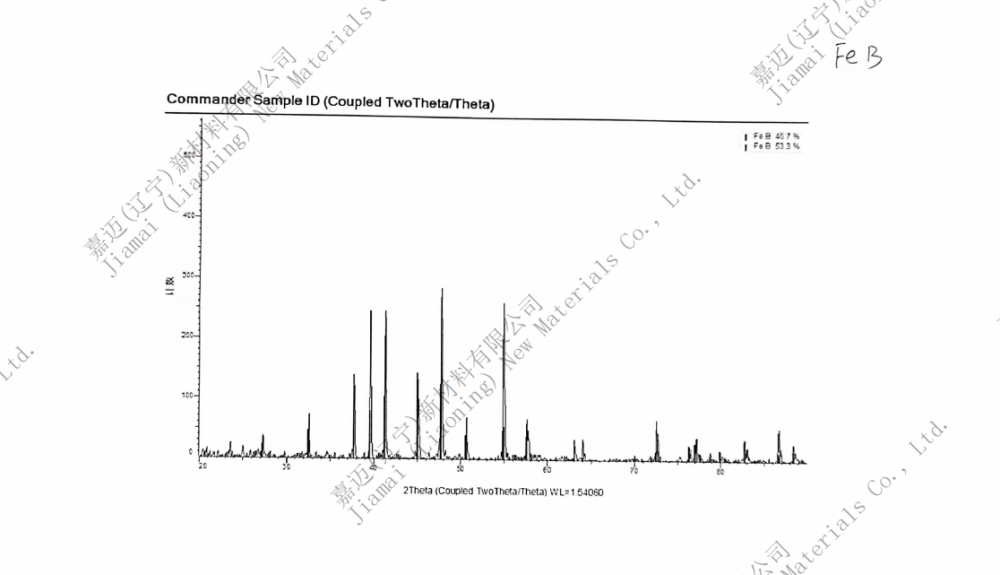


FEB
Molecular weight: 68.67188
Melting point: 1652 ℃
Density: 7.15g/ML (25/4 ℃)
CAS number: 12006-84-7
Production method:
Mix boron and iron in a molar ratio of 1:1 and prepare by co heating in argon at 1200-1300 ℃.
2. React FeS and BCl3 in hydrogen at temperatures above 500 ℃, or prepare them by reacting ferrous chloride solution with sodium borohydride (NaBH4).
Material structure:
The arrangement of iron boride is in a zigzag chain shape, with iron atoms at the corners of a triangular prism and boron atoms at the center of the triangular prism, covalently connected in the zigzag chain.
Application areas:
1) Boron iron has high hardness and good wear resistance, so it is widely used as a cutting tool and abrasive material.
2) Iron boride has a high melting point and excellent high-temperature resistance, making it an important component of high-temperature alloys and refractory materials
3) Boron iron also has good conductivity and magnetism, which can be used for the preparation of electronic components and magnetic materials.
4) Iron boride has a low coefficient of thermal expansion and good thermal conductivity, making it widely used in the preparation of high-precision instruments and thermal sensors.
5) Iron boride also has good chemical stability and can maintain good corrosion resistance in oxidizing environments, making it suitable for preparing corrosion-resistant materials.