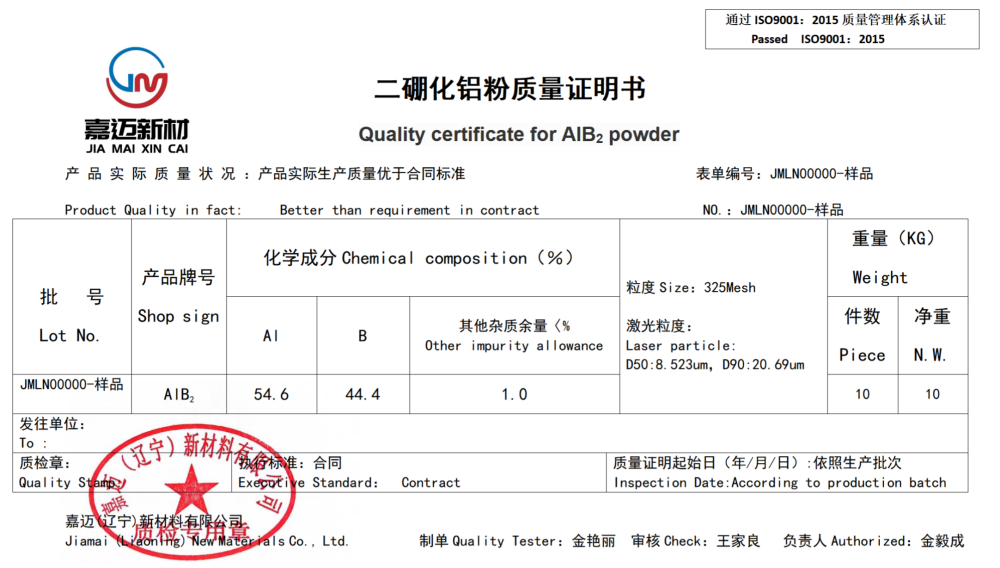
Product Name: Aluminum Diboride (AlB2)
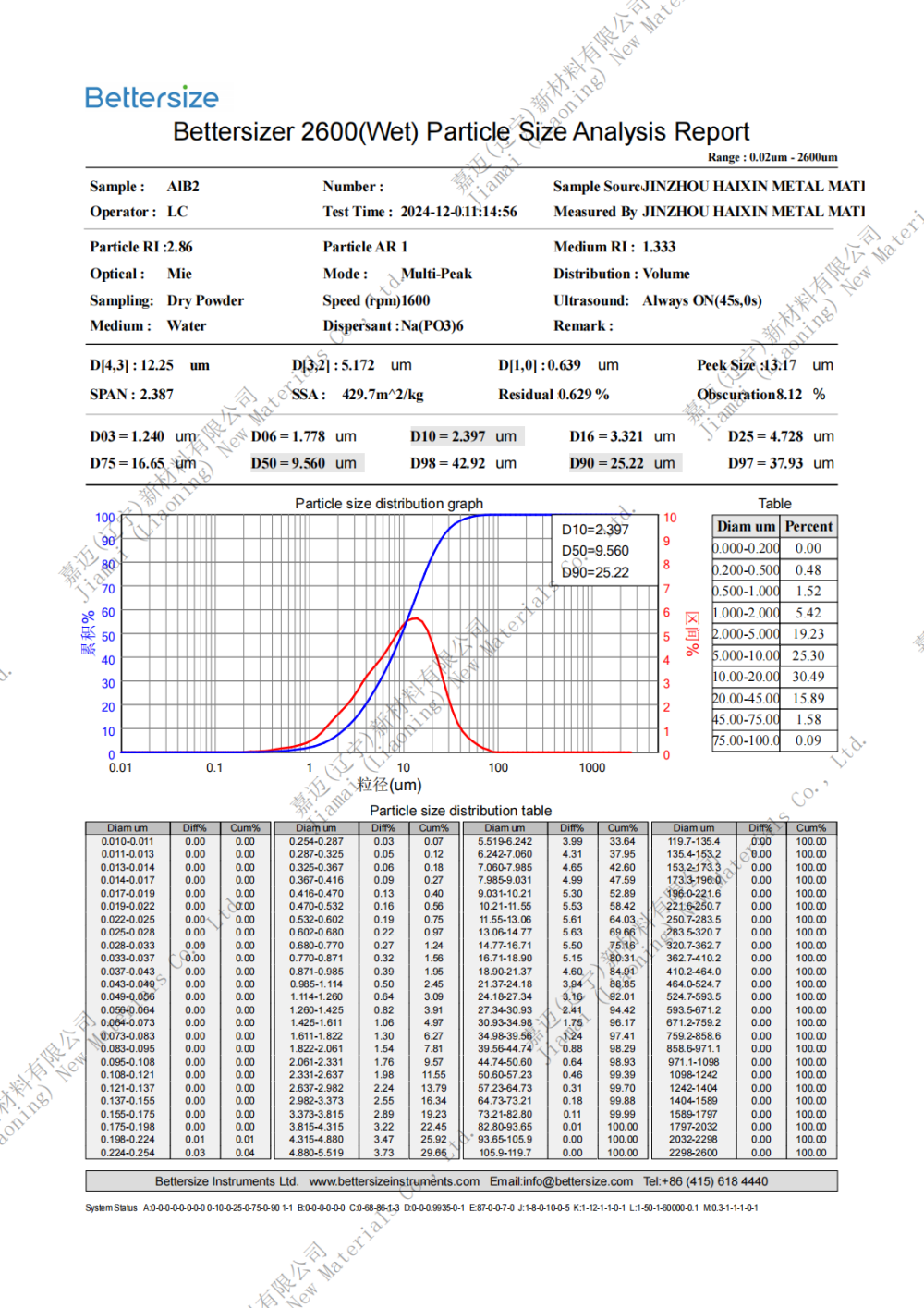
Specification: 0.8-10um (D50)
Appearance: Irregular
Color: Black Grey
Features: high hardness, high melting point, high thermal stability, excellent thermal conductivity
Usage: Aerospace, automotive, electronics, mechanical processing, heat dissipation devices, microwave communication, RF applications, catalysts and other fields


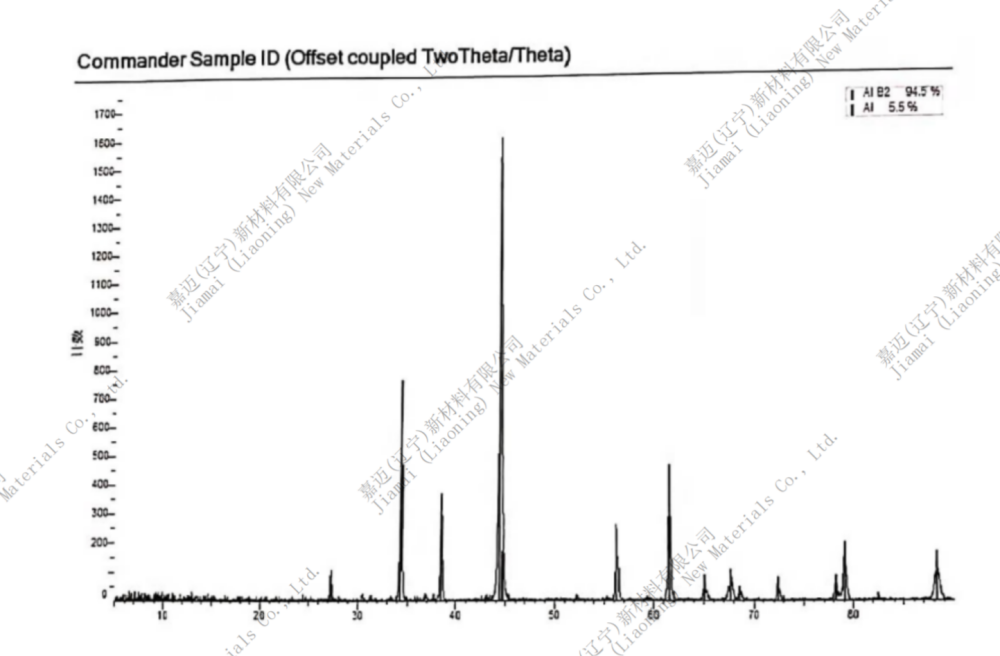


Aluminum boride (AlB2) is a binary compound formed by aluminum and boron elements. It is a gray red solid at room temperature and pressure, loses its surface gloss when heated, and is stable in cold dilute acid. It decomposes in hot hydrochloric acid and nitric acid. It is obtained by heating and reacting fine powders of aluminum and boron. It is one of the two compounds of aluminum and boron, the other being AlB12, which are commonly referred to as aluminum borides. AlB12 is a black, glossy monoclinic crystal with a specific gravity of 2.55 (18 ℃). It is insoluble in water, acid, and alkali and decomposes in hot nitric acid. It is obtained by melting boron trioxide, sulfur, and aluminum together. Structurally, B atoms form graphite like flakes with Al atoms between them, which is very similar to the structure of magnesium diboride. The single crystal of AlB2 exhibits metallic conductivity along the axis parallel to the hexagonal plane of the substrate. Boron aluminum composite materials are aluminum or aluminum alloys reinforced with boron fibers or boron fibers with protective coatings. The volume content of boron fibers is generally about 45% to 55%. Low specific gravity, high mechanical performance. The longitudinal tensile strength of unidirectional reinforced boron aluminum composite materials varies from 1.2 to 1.7 GPa depending on the process and raw materials, and the elastic modulus is approximately 200 to 240 GPa. The longitudinal specific modulus of elasticity and specific strength are approximately 3-5 times and 3-4 times that of titanium alloy hard aluminum and alloy steel, respectively. Good fatigue performance, with high strength even below 400 ℃, has been applied to turbojet engine fan blades and structural components of aviation, aerospace vehicles, and artificial satellites. Manufacturing plates, profiles, and complex shaped parts using hot press diffusion bonding method; Continuous casting can also be used to manufacture various profiles.
Production method:
The structure of aluminum boride is similar to that of intermetallic compounds, and its structure mainly depends on the crystal structure of aluminum metal and boron rather than their valence relationship. Aluminum borides include AlB2, AlB4, and AlB12. The diboride AlB2 can be formed by reacting two simple elements above 600 ℃. It is a layered structure, with Al atoms directly overlapping (A, A mode), and B atoms filling the triangular columns formed by the direct overlap of Al atoms, that is, the boron layer is located between two aluminum layers. The boron layer has a similar structure to graphite, with boron atoms connected in a hexagonal network. Each B atom is 0.173 nm away from the other three B atoms, and there are six Al atoms connected to B, occupying the vertices of the triangular column. AlB2 can dissolve in dilute hydrochloric acid to produce a reducing solution, which may contain HB (OH)+3. AlB2 is insoluble in dilute sulfuric acid, but can be dissolved in nitric acid. AlB2 decomposes to form AlB12 above 920 ℃.
Product performance:
The structure of aluminum boride is similar to that of intermetallic compounds, and its structure mainly depends on the crystal structure of aluminum metal and boron rather than their valence relationship. Aluminum borides include AlB2, AlB4, and AlB12. The diboride AlB2 can be formed by reacting two simple elements above 600 ℃. It is a layered structure, with Al atoms directly overlapping (A, A mode), and B atoms filling the triangular columns formed by the direct overlap of Al atoms, that is, the boron layer is located between two aluminum layers. The boron layer has a similar structure to graphite, with boron atoms connected in a hexagonal network. Each B atom is 0.173 nm away from the other three B atoms, and there are six Al atoms connected to B, occupying the vertices of the triangular column. AlB2 can dissolve in dilute hydrochloric acid to produce a reducing solution, which may contain HB (OH)+3. AlB2 is insoluble in dilute sulfuric acid, but can be dissolved in nitric acid. AlB2 decomposes to form AlB12 above 920 ℃.
Storage conditions:
This product should be sealed and stored in a dry and cool environment, and should not be exposed to air for a long time to prevent moisture from causing aggregation, affecting dispersion performance and use effectiveness. In addition, it should be avoided from heavy pressure, and should not come into contact with oxidants. It should be transported as ordinary goods.