
1. Introduction
In 2011, Professor Yury Gogotsi and Professor Michel Barsoum [1] of Drexel University in the United States used hydrofluoric acid to chemically peel the ternary layered carbide Ti3AlC2. A new type of two-dimensional carbide crystal Ti3C2Tx (T stands for functional groups such as -F and -OH) was successfully prepared (Figure 1).
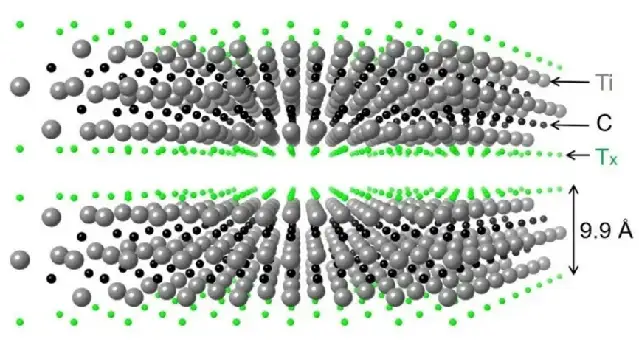
Figure 1 Schematic diagram of the atomic structure of the first Mxene material
Ti3AlC2 is a typical MAX phase. The MAX phase is A general term for a class of ternary layered compounds with a uniform chemical formula Mn+1AXn, where M is an early transition metal, A is the main group element Ⅲ and Ⅳ, X is C or N, n = 1, 2, 3, etc. The structure of MAX phase is characterized by the alternating arrangement of M atoms and A atom layers, forming A close packed hexagonal layer structure, and X atoms fill the octahedral gap, wherein M-A bond has the characteristics of metal bond, and the force is weaker than M-X bond.
Therefore, in hydrofluoric acid solution, the A atomic layer of the MAX phase is easy to be etched, leaving M and X atomic layers to form two-dimensional Mn+1Xn atomic crystals, in order to emphasize that they are stripped from the MAX phase and have a two-dimensional structure similar to Graphene, they are uniformly named MXene. Depending on the combination of various transition metals such as Ti, Mo, V, Cr and their alloys with C and N, materials scientists have identified or predicted the stable phases of hundreds or thousands of different MXenes.
Experimental and theoretical studies show that these materials have excellent mechanical, electrical, optical and electrochemical properties, showing excellent energy conversion and electrochemical storage potential, and have great application potential in the fields of lithium/sodium ion batteries, supercapacitors, photocatalysts, solar energy utilization, biomedicine and sensors.
As MXenes materials become more and more hot, they frequently appear in top journals such as Science, Nature, JACS, AM, etc. The application of MXenes materials also increases year by year with the growth of MXenes family, and the number of journal publications involving MXenes materials also increases rapidly. This indicates that research on MXenes has been in full swing in recent years (Figure 2).
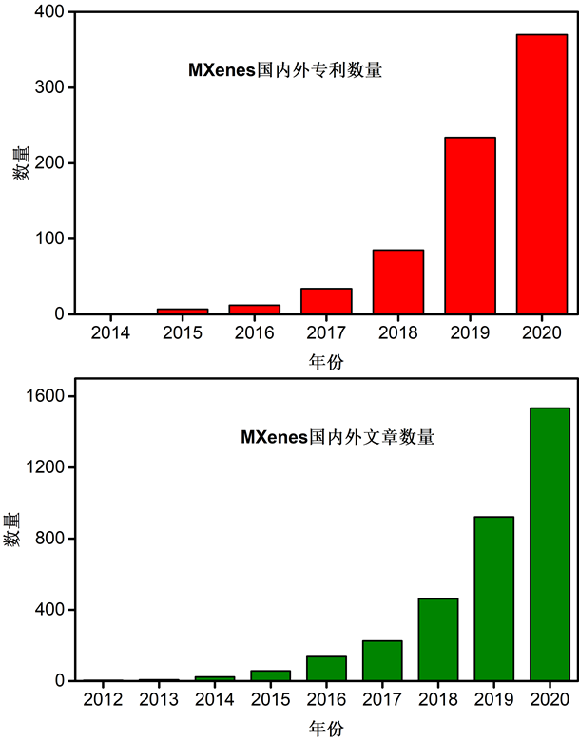
Figure 2 Statistics on the number of MXENes-related articles and domestic and foreign patents from the website "Web of Science"
As we all know, in the past few years, graphene has been the most promising material in the field of two-dimensional materials research. However, the practical application of graphene is limited by its simple chemical composition, as it only contains carbon networks. In addition, due to the lack of different surface groups on graphene and its high cost, its development has been hindered. As a novel two-dimensional material, MXenes has a near-metal conductivity (reported as high as 9880 S/cm), combined with excellent hydrophilicity, which overcomes the defect of serious loss of conductivity when graphene is oxidized or surface modified, so MXenes has been warmly "sought after" by researchers in various countries. It is gradually becoming the most eye-catching two-dimensional material in the 21st century.
2. Preparation method of Mxenes material
With the growth of MXenes family, the research on MXenes materials is also increasing. Figure 3 shows a brief timeline of progress in the discovery of MXenes materials. It can be seen that in recent years, the development speed of MXenes materials has become more and more rapid, and it can be seen from the figure that the process of preparing MXenes materials has become more and more abundant, gradually developing from the use of highly toxic and highly corrosive HF solution to more abundant, greener and healthy methods and processes such as melting salt, high-temperature alkali solution and electrochemical methods [2].
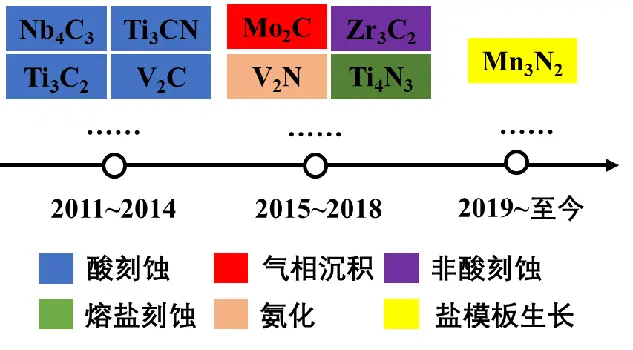
FIG. 3 Preparation process of Mxenes material
Fluorine-containing solution etching: Currently, F-ion containing solution is the most commonly used etching agent for the preparation of MXenes, and fluorine-containing etching agents can be mainly divided into two categories: acidic solutions containing fluoride ions (such as HF solution, LiF and HCl mixture or NH4HF2 solution) and fluorine-containing salt solutions (NH3F, KF, LiF or NaF). First, the MAX phase is immersed in a solution of F- ions to break the M-A bond, and then MXene is separated from the mixture. In this process, a certain corrosion time is required and sufficient agitation is required. Initially, Naguib et al. synthesized the first carbon-based MXene by dipping Ti3AlC2 powder into a concentrated hydrofluoric acid solution. At the same time, considering the harm of HF solution to the human body and the environment, since then, scientists have successively developed the use of safer mixtures of LiF and HCl or NH4HF2 solution as etching agents, and successfully prepared different MXenes materials.
Alkali solution chemical etching: The solution containing fluoride ions is the most effective chemical etching agent for preparing MXenes materials by the MAX precursor system. Although this method is effective, it has the following disadvantages: it is harmful to human body and environment; And the inert fluorine functional group will reduce the material properties (e.g., capacitance, etc.); In addition, HF solution corrodes not only Al layer, but also transition metal elements in MXene structure. What's more, certain etching byproducts are insoluble in any solvent under mild conditions and are difficult to remove from the prepared MXene. Therefore, there is an urgent need for A new fluoride-free method to remove the "A" layer atoms. It has been found that when a solution containing fluoride ions is used, the fluoride ions attack and remove the alkaline or amphoteric elements rather than the acidic elements. Therefore, A solution containing fluoride ions can synthesize MXenes materials by etching the MAX phase where most of the "A" atoms are Al and Ga. Due to the strong binding ability of alkali and amphoteric element Al, it is theoretically feasible to use alkaline solution to etch MAX with Al element in the "A" layer.
Electrochemical etching: The scientists demonstrated that the corresponding MXenes (Ti2CTx) phase can be prepared from Ti2AlC by electrochemical method in aqueous solution of HCl. In contrast to chemical etching methods using HF or a mixed solution of LiF and HCl, this electrochemical etching method does not contain any fluorine ions during the etching process, and the surface of the prepared MXenes will contain only -Cl, -O, and -OH groups. Further research revealed that Ti2AlC would be electrochemically etched into a three-layer structure. From the outside to the inside, this structure consists of carbide-derived carbon, MXenes, and unetched MAX. Moreover, MXenes can be further separated from this three-layer structure by ultrasonic method to obtain pure MXene. This result successfully proves that electrochemical etching can selectively remove layer A from the MAX phase without the use of fluoride ions to form MXenes materials without -F group, so the method also has potential application prospects.
Fused salt etching: Late transition metal halides (e.g. ZnCl2) are Lewis acids in their molten state, and these fused salts can produce strong electron-accepting ligands, which in turn can react thermodynamic with the A element in MAX. At the same time, the surrounding Zn atoms or ions can diffuse into the two-dimensional atomic plane and bond with the unsaturated Mn+1Xn nanosheets to form the corresponding MAX phase. The excess ZnCl2 can then etch the newly formed MAX to produce MXene. Several novel MAX phases (such as Ti3ZnC2, Ti2ZnC, Ti2ZnN, V2ZnC, etc.) and corresponding MXenes have been successfully synthesized by this method.
Although the current method of preparing two-dimensional MXenes materials can be mass-produced, the performance of MXenes materials is seriously affected by a large number of functional groups generated during the etching process, such as MXenes without functional groups are metallic, while MXenes with functional groups are semiconductors. Therefore, how to prepare pure MXenes materials is an important research direction in the future. In addition, the structure and morphology of MXenes materials prepared by different methods are different, and appropriate methods should be reasonably selected according to the needs.
3. Application analysis
The unique physical and chemical properties of MXenes materials have attracted extensive attention in recent years in many fields such as energy storage and conversion, sensors, biomedicine and multifunctional polymer composites. Figure 4 shows the common applications of MXenes, in order to further explain it, the author has made a detailed summary of some of the "hot" application fields.
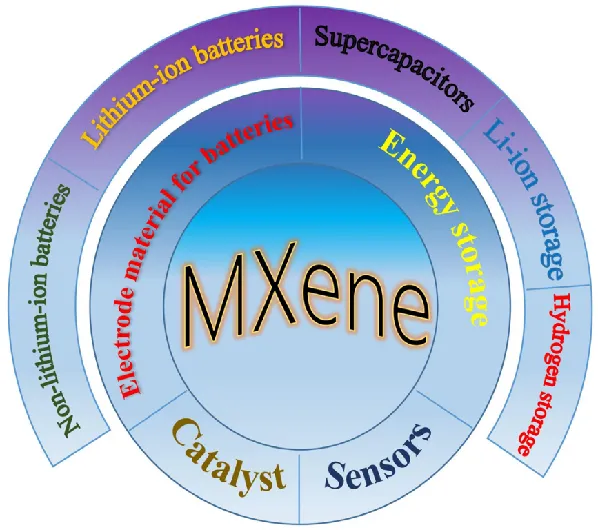
Figure 4 Common application areas of Mxenes materials
3.1 Energy Storage
Rechargeable secondary batteries have obvious competitive advantages in alternative energy storage, showing high theoretical capacity and energy density. The use of low-cost Earth-rich sodium or potassium ions to form Na ion batteries (SIB) or K-ion batteries (PIB) is expected to replace existing lithium-ion batteries (LiBs). However, its cell performance remains unsatisfactory due to the slow kinetics during the electrochemical reaction, which is due to the large ionic radius of Na+ and K+ and the severe volume expansion associated with their insertion and extraction. Therefore, there is an urgent need to explore high-performance electrode materials to facilitate the movement and storage of larger alkali metal ions, improve battery performance, and reach the standards required for commercialization.
Han Wei et al. [3] from Jilin University designed MXene@N doped carbon nanofiber structure as the anode of high performance sodium ion and potassium ion battery through in-situ bioadsorption strategy. The Ti3C2Tx nanosheets were assembled onto Aspergillus Niger to generate fungal nanoribbons, which were converted into 2D/1D heterostructures. This microbially derived two-dimensional MXene-1D nitrogen-doped carbon nanofiber structure has fully open pores and transport channels with high reversible capacity and long-term stability. It can store Na+(349.2 mAh g-1 1000 times in 0.1A g-1 cycle) and K+(201.5 mAh g-1 1000 times in 1.0 A g-1 cycle) (Figure 5). Ion diffusion kinetic analysis and density functional theory calculations show that this porous hybrid structure promotes the conduction and transport of Na and K ions, making full use of the inherent advantages of two-dimensional materials. Therefore, this study expands the potential of MXene materials and provides a good strategy for solving the challenges of two-dimensional materials in the field of energy storage.
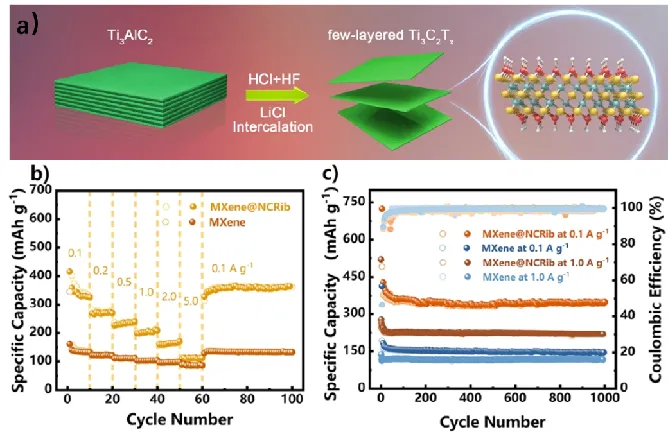
FIG. 5 Synthesis and electrochemical properties of MXene electrode materials
3.2 Catalysis
Photocatalytic water cracking holds promise for large-scale and sustainable solar hydrogen production. The earliest used semiconductor materials have low hydrogen production activity, and the recent development of Pt co-catalysts has effectively improved the hydrogen production activity and stability of photocatalysts, but the cost is high. Therefore, the development of highly active, abundant and low-cost co-catalysts to achieve clean and sustainable hydrogen production is an honorable and urgent task.
So far, cocatalysts with high activity, abundant reserves and low cost have at least the following problems: 1) It is difficult to establish a strong correlation between the surface of the cocatalyst and the surface of the photocatalyst, which is not conducive to the interface charge transfer and long-term stability. 2) The conductivity of the co-catalyst is poor or the π-conjugated system is damaged, resulting in low internal electron shuttle efficiency. 3) Gibbs free energy is not conducive to hydrogen evolution. 4) Insufficient hydrophilic function, resulting in insufficient contact with water molecules. 5) Stability is not enough, sometimes need to be in a non-water environment. As a new class of two-dimensional materials, MXenes shows great potential in solving the above problems: 1) The surface of MXenes contains a large number of -OH and -O, which can establish strong correlation with a variety of semiconductor surfaces. 2) Good electrical conductivity contributes to efficient charge-carrier transfer. 3) The metal sites exposed at the end make MXene potentially more REDOX active than carbon materials. 4) Good hydrophilicity ensures adequate contact with water molecules. 5) Can be stable in water.
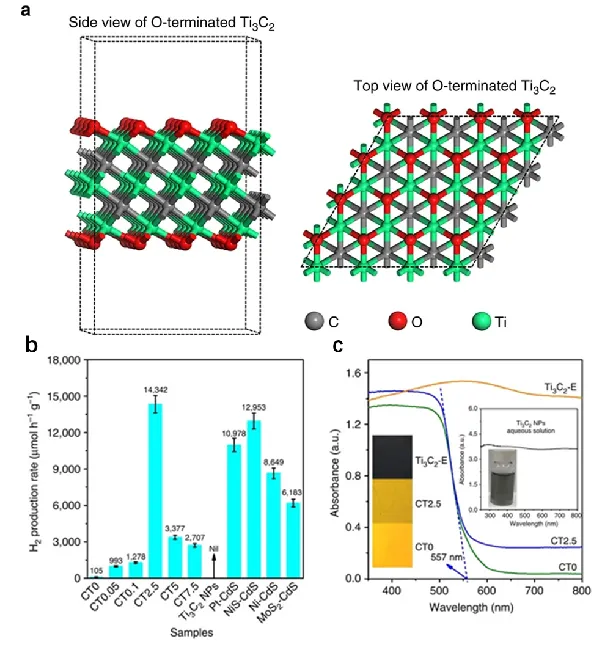
Figure 6. (a) Structural model; (b) Hydrogen production activity comparison; (c) UV-VIS diffuse reflection spectrum
As shown in Figure 6, Qiao Shizhang's research group [4] synthesized Ti3C2 nanoparticles, a MXene material with high cocatalytic performance, through DFT theoretical calculation. By hydrothermal integration of Ti3C2 nanoparticles onto the surface of the light-absorbing material CdS, the researchers achieved visible-light catalytic hydrogen production with an activity of up to 14,342 μmol h-1 g-1 and an apparent quantum efficiency of 40.1% at 420 nm (Figure 6b). The results of this study for the first time introduced MXene as a co-catalyst into the photolysis of aquatic hydrogen system, proving that MXene has great potential to replace Pt and construct low-cost, high-performance photoelectrodes or photocatalysts.
3.3 Optical Devices
Displays with self-luminous display characteristics have a wide range of application prospects, such as the OLED (organic light-emitting diode) display used by Apple mobile phones and the QLED (quantum dot light-emitting diode) display used by LG TVS are now commercially available. Unlike traditional LCD displays, this type of display technology requires no backlight and uses a very thin coating of organic, quantum dot or polymer materials and a glass substrate. When a current is passed through, these materials can self-light, so that the material can be lighter and thinner, greater viewing Angle, and can significantly save power consumption.
MXene is a two-dimensional transition metal carbide and nitride material with high electrical conductivity and optical transparency, and its shape is similar to potato chips stacked on top of each other. At present, this kind of material has been applied in many fields, such as energy, catalysis, medicine, etc., and has aroused worldwide attention. However, whether this material can be used in the field of light-emitting diodes has not yet been explored.
Professor Cheolmin Park [5] of Yonsei University in South Korea used MXene materials as polymer light-emitting diodes (PLED). As a solution-processable, large-area, flexible and transparent MXene-based PLED, it exhibits unprecedented high performance. As shown in Figure 7, under optimal AC conditions, the PLED switching voltage, current efficiency and brightness can reach 2.1V, 7CD A-1 and 12547 cd m-2, respectively. The excellent performance of MXene material on PLED is better than that of carbon nanotubes, reduced graphene oxide and Ag nanowires reported so far, which makes it very promising to become the next generation of flexible and transparent display materials.
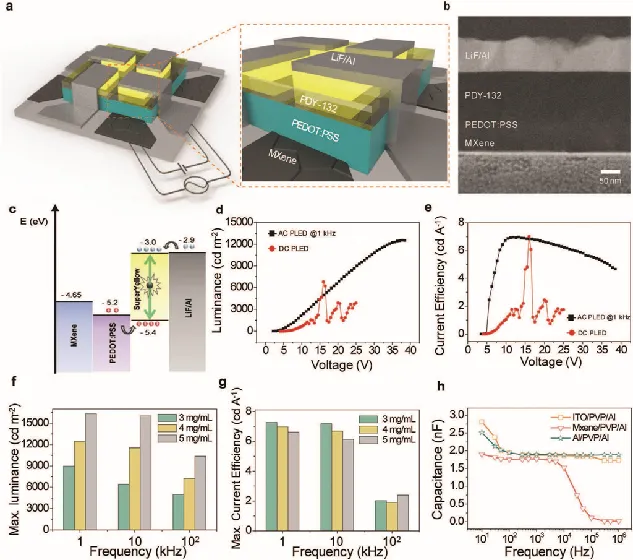
Figure 7. (a) Schematic diagram of MXene PLED; (b) TEM images of cross-section; (c) Energy level diagram; (d) L-V characteristics; (e) Current efficiency;(f-g) The relationship between maximum brightness and current efficiency and frequency; (h) Capacitance of different electrodes
3.4 Biomedicine
As of now, the COVID-19 pandemic remains a major global crisis. Although respiratory symptoms are a key feature of the disease, many people hospitalized with COVID-19 also suffer from acute kidney injury, a condition that exacerbates patient mortality and may have to be treated with continuous kidney replacement methods. During a pandemic, concerns about hospital capacity focus on the availability of ventilators. In this epidemic, however, hospitals are severely short of dialysis treatment supplies, including dialysate. Therefore, there is an urgent need to develop materials that can effectively and rapidly regenerate dialysate, remove toxins, and restore electrolyte concentrations in order for this important resource to remain adequately available.
The team of Professor Yury Gogotsi of Drexel University in the United States [6] used Ti3C2Tx, a MXenes material known to effectively adsorb urea, to remove creatinine and uric acid from aqueous solution and dialysate, with the maximum adsorption capacity of 45.7 and 17.0 mg g-1, respectively. The adsorption kinetics, isotherm and thermodynamics were systematically analyzed and simulated to determine the rate-limiting steps and adsorption mechanism, and a fixed bed post with Ti3C2Tx was designed to further evaluate the adsorption properties under continuous fluid flow conditions reflecting the conditions of continuous renal replacement therapy. The study shows that Ti3C2Tx has the potential to act as an effective adsorbent for dialysate regeneration, accelerate dialysate regeneration by removing filtered toxins, and realize more portable dialysis equipment manufacturing, reflecting the potential application of MXenes materials in the pharmaceutical field (Figure 8).
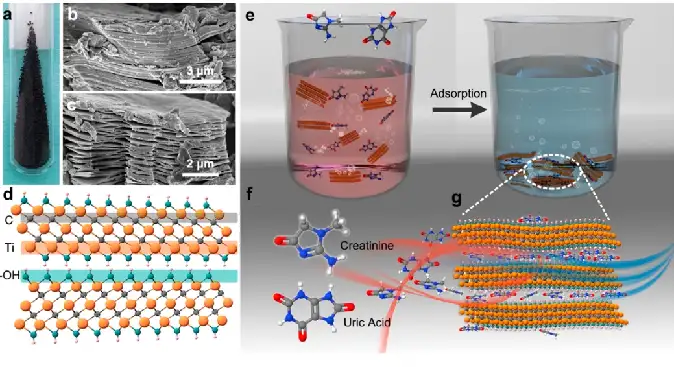
FIG. 8 Schematic diagram of Ti3C2Tx, creatinine and uric acid and their adsorption processes
3.5 Gas Sensing
Prevention is the best way to cure disease. Early detection, before the disease progresses, is likely to provide more opportunities for treatment, thereby increasing the likelihood of survival. One direction of early prevention is continuous physiological monitoring of the body from respiration, heart rate and skin. It is worth noting that about 200 compounds have been detected in human breath, and some of these gases can reflect the health of the body. Therefore, the use of gas detection and accompanying breath analysis will be a practical medical method.
At the same time, these gas sensors should be equipped with some other functions, such as portability, wearability, flexibility, and so on. However, existing conventional gas sensors are made on solid substrates, so they cannot be made into wearable electronic devices. In addition, metal oxide sensors that are now commercially available do not work at room temperature. Therefore, it is very necessary to develop new materials with good room temperature sensing properties on flexible substrates, and two-dimensional materials are theoretically predicted to have good room temperature gas sensing properties.
Professor Dong-Joo Kim [7] of Auburn University reported for the first time a gas sensor based on Ti3C2Tx, and used it to test ethanol, methanol, acetone, and ammonia. The Ti3C2Tx nanosheets were prepared on flexible polyimide films by easy solution casting, and their surface chemical properties were studied. As shown in Figure 9, the Ti3C2Tx sensor successfully detected ethanol, methanol, acetone, ammonia and other gases at room temperature, showing P-type sensing behavior. Because the Ti3C2Tx sensor has a large absorption energy, it has a high sensing response to ammonia gas. The possible sensing mechanism of the sensor is proposed based on the majority charge carrier transfer caused by the interaction between the sensing material and the sensing material. This new Ti3C2Tx gas sensor will be a new generation of general-purpose sensors for future wearable electronic devices, with performance comparable to other 2D material sensors, which also provides a new direction for the application of MXenes materials in sensors.
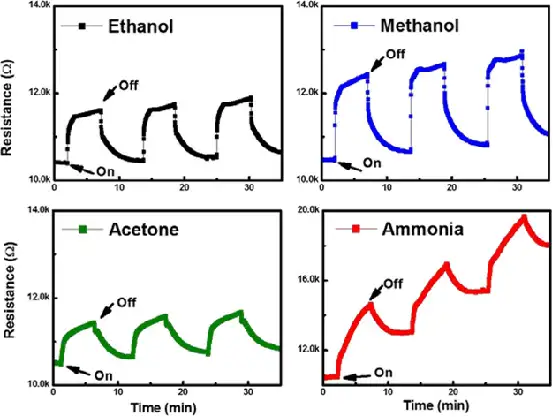
FIG. 9 Ti3C2Tx gas detection results at room temperature
4. Summary and outlook
Since its discovery, MXenes has been widely used in energy storage, catalysis and sensors due to its excellent electrical, optical, electrochemical and mechanical properties. After nearly ten years of extensive research, although great progress has been made in the preparation, structure and application of MXenes materials, there are still many problems in the research process.
For example, in terms of preparation: (1) Although the simulation and calculation of the structure and properties of different MXenes can provide guidance for the efficient preparation and practical application of MXenes materials, a large number of experiments are still needed to verify it; (2) Since the surface of MXenes prepared by chemical methods will be loaded with a large number of functional groups, there is still a long way to go in exploring more physical preparation methods (such as CVD method); (3) The currently prepared MXenes lamella is relatively thick, and the yield of single-layer or low-layer MXenes is very low, so it is necessary to seek more efficient preparation or separation methods to obtain MXenes materials with different layers and properties. There is still a long way to go.
In terms of application, although MXenes materials are very promising in many fields, due to the size effect, self-polymerization and stacking are easy to occur, therefore, greatly affecting the performance of the material. Although inorganic metal ions, organic molecules, polymer molecules and CNTs can be inserted between the two-dimensional nanomaterial sheets to reduce the self-polymerization of nanosheets, increase the active reaction site and improve the transport rate of electrolyte ions in the electrode material, the ion transport rate will decrease exponentially with the increase of the electrode material thickness.
Finally, MXenes is a new two-dimensional material with a variety of excellent properties. In its development and research process, it is not only necessary to develop more efficient preparation methods, but also to discover more other properties, and constantly explore more potential application fields.





